CE Marked Covid-19 IgM/IgG Antibody Rapid Test In Stock & Available To Buy
Product Code: 200081-4-2-3L
Size: 20 Tests
For medical healthcare professional in vitro diagnostic use only
COVID-19-CHECK-1 IgG/IgM Cassette (CE Marked 10min Whole Blood/Serum/Plasma) is a rapid immunochromatographic screening test for the qualitative detection of the anti-SARS-CoV-2 IgG/IgM antibodies in serum, plasma or whole blood. It is intended to be used by medical healthcare professionals as a tool to assess the COVID-19 serological status of people and cannot be directly used to diagnose a coronavirus 2019 infection (COVID-19).
The method relies on a mixture of recombinant SARS-CoV-2 antigens labelled with gold particles and anti-human IgM and IgG antibodies bound to the membrane solid phase. The multiple epitope fusion SARS-CoV-2 proteins are produced synthetically according to the genetic information of the virus. This material is therefore 100% non infectious.
Who can run these tests?
The test should be only be used in accordance with the instructions supplied and results interpreted by or under the supervision of a Medical Healthcare Professional. The test is not for home use.
Who can buy these tests?
These tests are only available to customers who have either a valid VAT number or a Company Registration Number and can place orders via formal purchase order from an official company domain. Customers will have to submit a statement to confirm that the test will only be used in accordance with the instructions supplied and results interpreted by or under the supervision of a Medical Healthcare Professional. The test is not for home use.
What does it do?
The test detects the presence of specific antibodies related to COVID-19 in a sample of whole blood, serum or plasma. The test will show a positive result if it is able to detect the presence of either of 2 different antibodies: IgM and IgG.
If an individual is exposed to COVID-19, IgM is the first antibody to be produced in order to immediately help the human body to fight an infection. There is a delay of approximately 8 – 11 days* post infection before the IgM antibody is produced to a high enough level whereby it is detectable by testing which means that a test could return a negative result (see Limitations section). However, once this detectable level has been reached, when a test is performed it will return a positive result.
The IgG antibody is produced after the initial phase of the infection to help protect against a repeat infection. This is detectable approximately 18 – 21 days* post viral infection. Once a detectable level of IgG has been reached, when a test is performed it will return a positive result.
If just IgM is positive then it is likely that the disease is active. If the result is IgG positive, or both IgG and IgM positive, that suggests that the subject may have evidence of some immunity. A positive IgG test does not guarantee immunity to COVID-19, but it is an indication of exposure. See figure 1 Approximate IgM and IgG antibody levels over time – Not actual data – for illustration purposes only
If the test is negative for both IgM and IgG antibodies this means that the patient could be in the latent phase of the infection or has not been infected.
*NOTE: The time frame for detection of both IgM and IgG antibodies can vary depending upon which publications are referenced and the cohort of patients that were tested.
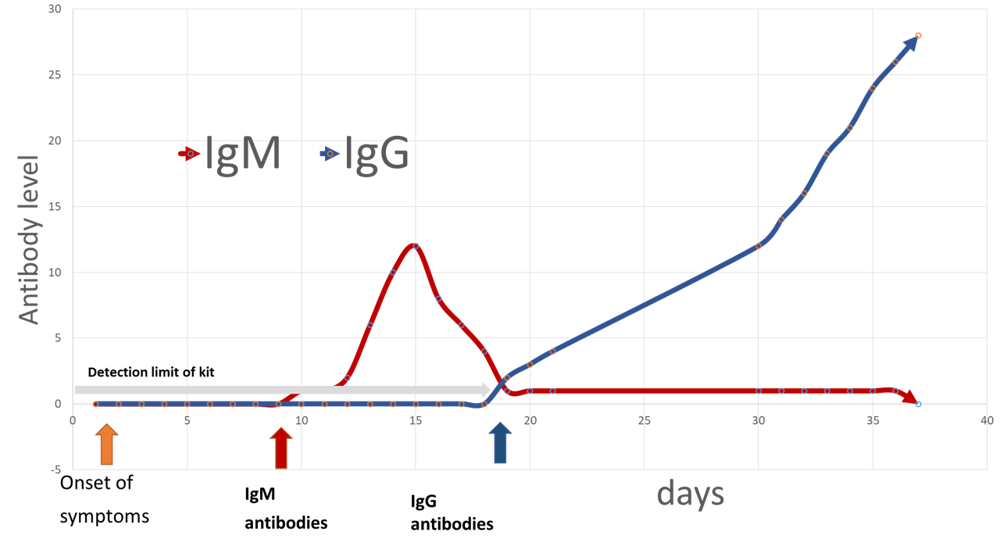
Fig.1 – Approximate IgM and IgG antibody levels over time – Not actual data – for illustration purposes only
What is the difference between IgG and IgM antibodies?
Immunoglobulin G (IgG), the most abundant type of antibody, is found in all body fluids and protects against bacterial and viral infections. Therefore, this is the antibody that would be expected to found in the human body to help protect against repeat infection COVID-19.
Immunoglobulin M (IgM), which is found mainly in the blood and lymph fluid, is the first antibody to be made by the body to fight a new infection. Therefore, this is the antibody that would be expected to found in the human body to help fight an initial infection of COVID-19.
What is the difference between Primary SARS-CoV-2 infection and Secondary SARS-CoV-2 infection?
Primary SARS-CoV-2 infection is characterised by the presence of detectable IgM antibodies approximately 8 – 11 days* post infection.
Secondary SARS-CoV-2 infection is characterized by the elevation of SARS-CoV-2 specific IgG. In the majority of the cases, this is accompanied by elevated levels of IgM.
What are the possible results?
IgM POSITIVE:* Two lines appear. One coloured line should be in the control line region (C), and a coloured line appears in IgM test line region (T1). The result suggests the sample contains IgM antibodies specific to the SARS-CoV-2 and indicates a primary/recent infection.
IgG POSITIVE:* Two lines appear. One coloured line should be in the control line region (C), and a coloured line appears in IgG test line region (T2). The result suggests the sample contains IgG antibodies specific to the SARS-CoV-2 and may indicate a secondary infection or late stage of the disease.
IgG and IgM POSITIVE:* Three lines appear. One coloured line should be in the control line region (C), and two coloured lines should appear in the IgM test line region (T1) and the IgG test line region (T2). The colour intensities of the lines do not have to match. The result suggest the sample contains both IgM and IgG antibodies specific to the SARS-CoV-2 and may indicate an active or a secondary infection.
*NOTE: The intensity of the colour in the IgG and/or IgM test line region(s) will vary depending on the concentration of SARS-CoV-2 antibodies in the specimen. Therefore, any visible line in the test area either strong or weak (even much weaker than the control line) should be interpreted as positive.
NEGATIVE: One coloured line should be in the control line region (C). Thie result suggests the sample does not contain detectable IgM nor IgG directed against SARS-CoV-2.
INCONCLUSIVE: If there is no distinct colour band visible in the control zone (C), the test is inconclusive. It is recommended, in this case, to repeat the test with a new device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
Please note that individuals’ coexisting conditions need to be taken into consideration. People who have suppressed immunity due to illness or immunosuppressing drugs may not produce antibodies in sufficient levels to be detected by this method.
What are the Limitations?
1. The COVID-19-CHECK-1 procedure and the interpretation of results must be closely followed. This assay is designed for detecting antibodies against SARS-CoV-2 in human serum, plasma and whole blood. Any results derived from the assay of other body fluids and from assay of pooled serum, plasma or whole blood may not be interpreted correctly, based on the current criteria. So, other body fluids and pooled samples cannot be assayed using COVID-19-CHECK-1.
2. For asymptomatic patients or when signs are not clearly interpretable, but a COVID-19 case is suspected, it is recommended to repeat the testing, in case of first negative result, 2 or 3 weeks later or to perform a PCR test.
3. As for any diagnostic procedure, the physician should evaluate the data obtained using this kit in the light of the other clinical information available.
4. COVID-19-CHECK-1 is a qualitative test that cannot be used to determine the anti-SARS-CoV-2 antibody concentration nor the rate of increase in antibody level.
5. An excess of biotin (vitamin B8) can lower the control line colour intensity or even lead to the control line disappearance if the biotin concentration level is very high.
6. SARS-CoV-2 infection in pregnant women may increase health risks to both mothers and infants during pregnancy.
7. In the early stage of the disease, IgM level could be below detectable levels.
8. The presence or the absence of antibodies cannot be used to determine the success or failure of a medical treatment.
9. Results in immunosuppressed patients should be interpreted with caution and need a medical advice.
10. If the test is negative and clinical symptoms persist, additional testing using other clinical methods is recommended. A negative result does not preclude the possibility of SARS-CoV-2 infection.
11. In the early onset of fever, anti-SARS-CoV-2 IgM concentrations may be below detectable levels. IgM may appear within 3-7 days after infection and then the concentration may decrease while IgG concentrations may increase. A positive result for IgM is indicative of primary SARS-CoV-2 infection, however a negative result does not at any time preclude the possibility of SARS-CoV-2 infection. Therefore IgM readings should be treated as advisory and should not be used as the sole criteria for the diagnosis of SARS-CoV-2.
12. COVID-19-CHECK-1 will only indicate the presence of SARS-CoV-2 antibodies in the specimen and should not be used as the sole criteria for the diagnosis of SARS-CoV-2.
13. As with all diagnostic tests, all results must be interpreted together with other clinical information available to the Medical Healthcare Professional.
Is the COVID-19 IgG/IgM Cassette CE-marked?
Yes. The test is CE-marked for Medical Professional Use only in accordance with EU IVD regulations.
What samples are required to run the test?
The test works with either whole blood, serum, or plasma.
What is the testing time?
The test result should be read at 10 minutes. Do not interpret the result after 15 minutes.
Is there an order limit?
Kits are supplied in quantities of 20 tests.
For more information, register an interest or to request an IFU, please contact us.


